Archive for January, 2012
Embryo Cryopreservation: An easy to understand review
Why are Embryos Cryopreserved?
Infertility patients invest so much time, effort, money and emotion into each IVF cycle. After the fresh embryos are transferred, about 30-40% of our patients will have excess high quality embryos available for freezing, also known as cryopreservation, to be used for future cycles. Frozen embryo transfers are a cost-effective way to pursue further treatment, regardless of the outcome of the fresh embryo transfer cycle.
Patients are often curious about the methods their laboratory uses to freeze their embryos. Cryopreservation is a delicate process requiring extensive expertise to prevent damage to the embryos during the freezing and thawing procedures.
Preventing Potential Damage During Freezing
There are three major types of injury that can occur to cells during the cryopreservation procedure (Jain JK, et al., 2006):
- (i) Exposure of cells to ice crystal formation during freezing and/or thawing;
- (ii) Damage to the embryos from the solutions used to prepare the embryos prior to freezing; and
- (iii) Damage to the embryos as water and electrolytes shift in and out of the cells that make up the embryos.
Damage from ice crystal formation is overcome by removing as much water from within the cells making up the early embryo and replacing it with fluid containing cryoprotectants. To remove the water, embryos are placed in a series of solutions using increasing concentrations of salt to draw the water out of the cells. Once this is accomplished, the embryos are then placed into additional solutions containing the cryoprotectants, which enter the cells taking the place of the water.
Removing the water from the cells is relatively simple while replacing the fluids with cryoprotectants is a bit more difficult and damage to the embryos can occur if not done correctly. Cryoprotectants can be toxic to the embryos, so the cells can only be exposed to them for a very short time before they are frozen. Once the cells have been filled with cryoprotectants, cryopreservation needs to take place fairly rapidly.
Slow Freezing and Vitrification Techniques
The two most common cryopreservation methods used to freeze embryos are slow freezing and vitrification. The slow
freezing method has been around for decades. Slow freezing involves loading the embryos into special straws or vials and placing them in a separate container, which is surrounded by a liquid nitrogen bath. The container controls the rate of temperature drop and freezes the embryos slowly over a few hours, preventing most ice crystal formation. The freezing device lowers the temperature gradually to -38° C. Once the embryos have reached this temperature, the vial or straw is plunged into a bath of liquid nitrogen to reach a final temperature -196°C (-321°F). They will remain at this temperature until they are thawed.
With vitrification, embryos are rapidly cooled to -196°C (-321°F) almost instantly. This instant cooling does not allow ice crystals to form. Vitrification comes from the Latin vitreum, meaning the transformation of a substance to glass. Please note the use of the word “cooling” is used rather than “freezing” when referring to vitrification. Freezing actually involves transitioning a liquid to a solid through crystallization. No crystals are actually formed during vitrification. To understand the difference better, take a look at an ice cube from your freezer. While it is mostly clear, you will notice it is somewhat cloudy due to ice crystal formation and not as clear as glass. Water that is vitrified appears absolutely clear because ice crystals never had the chance to form and a glass-like substance is created.
Vitrification still requires the replacement of water with cryoprotectants but uses a different recipe. In fact, the cryoprotectants used for vitrification are at a higher concentration and potentially even more toxic to the embryos. Once the embryos are exposed to the high concentration cryoprotectants, they must be frozen very rapidly. Whereas the slow freeze process takes place over hours, vitrification takes place over minutes.
Is One Cryopreservation Technique Better than the Next?
The process of embryo vitrification is relatively new compared to the slow freeze method; however, great advances have been made over the past decade.
There is evidence that vitrification is a slightly better system than slow freezing. In most studies, the embryo survival rates are better for vitrification than the slow freeze technique. A recent study by Kaskar, K. et.al, also showed that the pregnancy rates with vitrified/warmed embryos (64%) were significantly higher than those that were frozen/thawed by the slow freeze techniques (47%). With higher survival rates and higher implantation rates, vitrification is slowly replacing the slow freeze technique, especially for the storage of eggs and embryos.
Success with Re-vitrification
Vitrification may allow embryos to be warmed, re-vitrified, and warmed again with successful pregnancies reported. While the pregnancy rates are somewhat lower that that of embryos vitrified only once, the results are far better than embryos slow frozen, thawed, re-frozen, and thawed yet again. A 2007 study by Kamasko, Y. et.al, showed no significant differences in implantation rates between once vitrified embryos and twice vitrified embryos. The reason this is important is that embryos may be frozen/cooled in groups larger than we want to transfer. For example, if a patient succeeded with a twin pregnancy with two fresh embryos transferred and only wants one frozen embryo transferred for a future child, having two cryopreserved embryos survive that were stored in a single vial would present a problem. In this case, we would possibly transfer more embryos than we wanted to, discard the extra embryo (not at all desired) or refreeze/re-vitrify the extra embryo. Using vitrification, the embryo may indeed survive a second stage of warming for an additional pregnancy in the future.
Your Embryos Are Very Important
As embryologists, we take our responsibilities to care for your cryopreserved embryos very seriously so that you will have the opportunity to use them for future treatment cycles. Rest assured they will be available when you need them, having been cryopreserved with the most state-of-the-art techniques at our disposal.
Thawing/Warming Your Embryos
If you would like to learn about how embryos are thawed/warmed, be sure to watch for our upcoming blog on this topic!
References:
Kaskar, K., et.al. Comparison of clinical outcome of blasocyst vitrification with slow freezing and fresh embryo transfer. Fertil Steril 2010;94:113-114Jain JK, Paulson RJ. Oocyte cryopreservation. Fertil Steril 2006;86(Suppl 3):1037-46.
Kamasko, Y. et.al. The efficacy of the transfer of twice frozen-thawed embryos with the vitrification method. Fertil Steril 2009;91:383-386
Do These Donated Embryos Make the Grade?
Embryo Grading Made Easy For Embryo Donors and Embryo Recipients
Part 1 of a 3 part mini-series by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D.
Embryo grading is an important factor for both donors and recipients. Potential embryo donors want to know if we will accept their embryos for donation since part of our decision is based on the grade of their embryos. Likewise, potential embryo recipients want to know how likely the donated embryos are to survive thawing and if they are of good enough quality to build their family. Part of our estimation of success depends on the grade of the embryos.
We wish this process could be easier since there is no standardized system used in all embryology laboratories to grade embryos. Furthermore, the grading of embryos is somewhat subjective so one embryologist may grade an embryo
somewhat differently than a colleague.
Embryo grading is an imperfect process; poorly graded embryos may occasionally result in ongoing pregnancies and beautiful looking embryos may not implant and grow. Poorer graded embryos will not necessarily result in an abnormal child; they simply seem to implant and grow less frequently.
So, the appearance and grading of an embryo is an imperfect estimate of the quality of the embryo as well as the embryo’s true potential. It is, however, the best way we have to visually estimate the implantation and live birth rate of a given embryo. We will now examine one of the more common methods used to grade embryos.
When are embryos graded and cryopreserved?
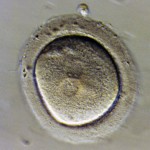
Embryos are usually graded and frozen at three specific stages with “Day 0” being the day of retrieval and fertilization:
| Age of Embryos | Day 1 | Day 3 | Day 5-6 |
| Common terminology | 2PN (pronuclear stage)
2 cell stage |
Embryos are 6-10 cells | Morula &
Blastocysts |
| Grading importance | Grading not available | Grading relatively important | Grading very important |
| How often these are sent to EDI? | Rarely sent | 40% of EDI embryos | 60% of embryos |
Embryos cryopreserved immediately after fertilization are confirmed early on Day 1 (the 2PN (pronuclear stage), but aren’t advanced enough to be consistently graded. Freezing embryos on Day 1 is quite infrequent unless we are certain there will not be an embryo transfer. Accordingly, these embryos are rarely sent to EDI for embryo donation.
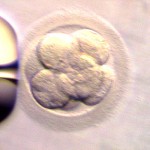
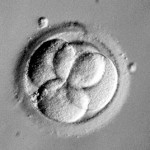
Grading Day 3 Embryos
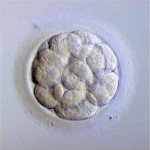
Day 3 embryos are graded on cell number, the amount of cellular fragmentation and the symmetry of the embryo.
Cell Number
Most Day 3 embryos will be comprised of 6-10 cells called blastomeres. Embryos with too few blastomeres may not be healthy, so we prefer at least 7-8 at this stage. Embryos with fewer cells may not be healthy growing very slowly or may have stopped growing entirely commonly called “cell block”.
Fragmentation
Fragments may be found in many of the embryos, which are “bits” of cells that break off from a blastomere. We prefer as little fragmentation as possible. Fragmentation is estimated as the percentage of fragmentation volume compared to the total embryo volume and is converted to a letter grade in the following way:
- 0 % = A
- 1-10% = B
- 11-25% = C
- >25% = D
A large amount of fragmentation may be caused by death of one or more of the blastomeres. The higher the fragmentation, the lower the quality of the embryo & letter grade and the less likely that the embryos will survive thawing. Embryos with high fragmentation rates implant less frequently when transferred fresh or when thawed.
Symmetry
Symmetry of the embryo refers to the shape of the individual blastomeres and the overall shape of the embryo. The blastomeres should all be very similar in size and generally round in shape. The scale used to grade symmetry is
- Perfect = A
- Moderately asymmetric = B
- Severely asymmetric = C
Severe blastomere asymmetry (i.e., large and small blastomeres in the same embryo) reflect nuclear/chromosomal and cytoplasmic problems suggesting the embryo is less healthy than desired. There is supporting evidence that blastomere symmetry is important and reflects overall health of the embryo. Interestingly, the overall symmetry of the embryo (round vs. oval) is of uncertain importance with some very “funny looking” embryos resulting in beautiful and healthy children.
Putting it All Together for Day 3 Embryos
For Day 3 embryos, the order of grading is the “number of cells (#c),” “fragmentation grade” and “symmetry grade”. For example:
- 7cAA = 7 cells with no significant fragmentation and perfect symmetry
- 8cBA = 8 cells with 1-10% fragmentation and perfect symmetry
- 6cBB = 6 cells with 1-10% fragmentation and moderate asymmetry.
Grading Day 5 Embryos
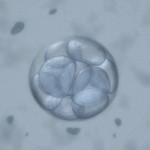
More advanced embryos are graded and potentially frozen on Day 5 or Day 6. These are generally described as morula or blastocysts.
Day 5 Morula Embryos
Morula embryos are difficult to grade as the cells combine, forming essentially a ball of cells that can’t really be categorized in any way other than descriptive terms:
- Morula (early)
- Compacting morula (more advanced)
While some facilities only occasionally cryopreserve Day 5 morula embryos, it is thought that the survival and implantation rates of these embryos may be slightly reduced but they are still quite reasonable, suggesting that they should not be discarded. Day 6 morulas are probably delayed in growth or may have stopped growing, may not be viable and are infrequently cryopreserved.
In order to balance the possible reduced implantation rates, it is common that more morula embryos are thawed and transferred in order to achieve success.
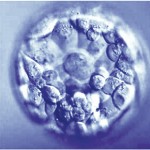
Day 5 Blastocyst Embryos
Day 5 blastocyst embryos are the most advanced embryos we see in IVF. These embryos are formed within 24 hour of actual implantation. Trying to grow embryos beyond this point is
technically difficult, as the embryos usually don’t survive. In addition, the window of time for implantation seemingly closes beyond Day 5 or early day 6. For example, transfer on Day 7 will rarely result in implantation. So, it simply makes more sense to transfer and/or freeze blastocysts rather than trying to grow them any further.
Along with descriptive measures, more objective grading is attempted through evaluation of the cellular expansion, the inner cell mass (which eventually becomes the fetus) and the quality of the outer cell mass called the trophectoderm (which eventually forms the membranes and placenta).
Expansion
As the embryo advances in growth, a cavity called the blastocoel fills with fluid. As the cells continue to divide and the fluid collects, the embryo expands and eventually escapes its outer covering called the zona pellucida. The blastomeres continue to group together wherein the individual cell cannot be counted. As the Day 5 embryo expands, differentiates and escapes the outer zona pellucida, the grade increases numerically from 1-5.
| Grade | Description | Physiology |
| 1 | Early Blastocyst | Starting to form a fluid-filled space in the middle (Blastocoel). Grading the embryo is difficult here. |
| 2 | Full Blastocyst | Blastocoel forms and inner cell mass is now distinguishable. Grading can be done from this point forward. |
| 3 | Expanded Blastocyst | Blastocyst is starting to expand in size thinning the outer covering, the zona pellucida |
| 4 | Hatching Blastocyst | Blastocyst is starting to hatch out of the zona pellucida. |
| 5 | Hatched Blastocyst | Blastocyst is fully hatched and now ready for implantation into the uterine wall. |
Inner Cell Mass (ICM)
As the blastomeres compact to form the inner cell mass (ICM), this early fetal tissue is graded on a A-D scale:
| ICM Grade | Description |
| A | ICM with total compaction |
| B | ICM still compacting |
| C | Reduced ICM |
| D | Poor with dying cells |
Trophectoderm (TE)
The outer cells of the trophectoderm (TE) also reflect the overall health of the embryo and are graded in an A-D scale.
| TE Grade | Description |
| A | Numerous cells forming cohesive layer |
| B | Few but healthy large cells forming a loose epithelium |
| C | Few cells present often with asymmetric distribution |
| D | Poor with degenerating/dying cells |
Putting it All Together for Day 5 Embryos
For Day 5 embryos, the order of grading is “expansion,” “inner cell mass” and “trophectoderm.” For example:
- 1 = Early blastocyst is unable to be easily graded with respect to ICM or TE as these haven’t separated well enough yet.
- 2BB = Blastocyst with partial ICM compaction with loose, large trophectoderm cells
- 3AB = Expanding blastocyst with total compaction of ICM and with loose, large trophectoderm cells
- 4AA = Hatching blastocyst with excellent ICM & trophectoderm cell layers
- 5AA = Fully hatched blastocyst with excellent ICM & trophectoderm cell layers
Day 5 embryos that are graded 4AA and 5AA are some of our favorite embryos.
Summary Comments
Embryology laboratories strive to grow the healthiest embryos they can. Over time, they have adapted different grading techniques so the laboratories can communicate the quality of the embryos to physicians, patients and each other. Not all beautiful embryos will implant or produce a healthy child but they seem to do so more often than others. Not all poorly developing embryos will fail to implant and produce a healthy child but most do not result in live offspring.
At EDI, we try to only accept embryos that are likely to implant so embryos with less than a B rating for any category are infrequently accepted. This is done to assure our recipients that all of the donated embryos we offer are of the highest quality and provided the greatest chance for a successful pregnancy.
This is only one piece of the puzzle as many other factors influence the likelihood for success. Dr. Sweet will cover this topic in the separate blog within the next couple of months.
Also stay tuned to the upcoming blogs regarding how embryos are frozen and thawed and what techniques seem to work the best.
While embryo grading is not a perfect system, we use it to try to predict the overall quality of the embryos and their potential to survive thaw, grow and build a recipient’s family.
Corey Burke. B.S., C.L.S.
Laboratory Supervisor
CBurke@EmbryoDonation.com
Craig R. Sweet, M.D.
Reproductive Endocrinologist
Info@EmbryoDonation.com
References
Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, Pomeroy KO, Wininger D, Gibbons W, Conaghan J, Stern JE. Standardization of grading embryo morphology. Fertil Steril. 2010 Aug;94(3):1152-3.
New Mini-Series on How Embryos are Processed
 Beginning next week, we’ll be publishing a mini-series on how embryos are processed in the laboratory. Written by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D., the three-part series will cover how embryos are initially graded, frozen and finally thawed. The information is designed for patients and written in an easy to understand style.
Beginning next week, we’ll be publishing a mini-series on how embryos are processed in the laboratory. Written by Corey Burke, B.S., C.L.S. & Laboratory Supervisor and Reproductive Endocrinologist Craig R. Sweet, M.D., the three-part series will cover how embryos are initially graded, frozen and finally thawed. The information is designed for patients and written in an easy to understand style.
We hope this will be an informative series, answering many common questions alike. If you have additional questions, please feel free to post them as comments here, or after the specific blog post.
Thank you for reading and stay tuned for detailed embryo grading, freezing and thawing information starting with the first series post on Tuesday, January 10th.
–Embryo Donation International